
What is the issue?
Once a vessel is cast off from the dock all the power it requires has to be self-generated and then stored in batteries which require some working knowledge and maintenance.Why address this?
As the electricity stored in these batteries plays a pivotal role aboard a seagoing vessel, not only for our personal comforts but also for running key sailing equipment, such as the vessel's instruments, radar, self-steering, navigation lights, not to mention providing the required power to start the engine, it is essential that that these batteries are well maintained and can serve the boat and crew well. Diligent maintenance is also the key to a long battery life. The objective of this article is to provide a good first overview of the maintenance and optimal charging of batteries.How to address this?
There is an old saying that 'batteries don’t die, they are killed'. Good management practices are your insurance against early failure, regardless of the technology used. The basic principles of battery care revolve around:- • Check voltage at least once daily.
- • Keep batteries charged - methods include solar chargers, wind-powered chargers or battery chargers connected to some sort of shore power. It is good policy to make sure your batteries are fully charged on a monthly basis, this will minimise the likelihood of sulphating occurring.
- • Be aware of any gassing that may occur in flooded batteries and top up electrolyte levels as required.
- • Ensure that batteries are not drained below their safe operating level. For most lead acid deep discharge batteries (flooded, gel, Absorbed Glass Mat) this is around 50% but some battery types can be safely discharged further.
Batteries (Accumulators) Overview
 A small boat battery
A small boat batteryPhoto: Michael Harpur
Nearly all large lead-acid type batteries have the following properties:
- • They are made up of a number of cells which consist of two sets of lead plates separated by wooden or porous plastic separators and filled with a dilute sulphuric acid solution called the electrolyte. This applies only to Wet Cell (flooded) Lead Acid Batteries.
- • One set of lead plates (alternate plates) are the positive plates which form lead-peroxide and a chocolate-brown colour when the accumulator is fully charged.
- • The plates and separators are usually placed into a vulcanite, hard rubber, glass or plastic case sealed with pitch, through which the terminals protrude.
- • When discharged both the positive and negative plates form lead sulphate. This is caused by the chemical action during discharge when sulphur and oxygen are transferred from the electrolyte to the plates, reducing the specific gravity of the electrolyte.
Plates in a discharged condition are easily recognised by the appearance of lead sulphate (a white deposit) on both positive and negative plates. Upon recharging, the lead sulphate is transferred back to the electrolyte and the specific gravity increases provided the accumulator has not been left standing in a discharged condition for a long time when a hard core of lead sulphate forms. When the accumulators have been left in a discharged condition for too long the lead sulphate hardens and causes the plates to thicken and buckle, damaging the separators and casing and no longer function efficiently, if at all, in which case the batteries should be reconditioned or replaced.
Electrolyte
All lead-acid battery plates are immersed in a strong electrolyte, which provides a mechanism for a charge to flow between positive and negative plates. There are separators (insulators) between the positive and negative plates to prevent inadvertent contact. The electrolyte consists of a solution of sulphuric acid diluted to correct specific gravity by distilled water.
The electrolyte should just cover the plates and maintained at that level by the additional of pure distilled water when the level drops below the top of the plates. Many batteries are now sealed and so can't be topped up.

Testing Battery State
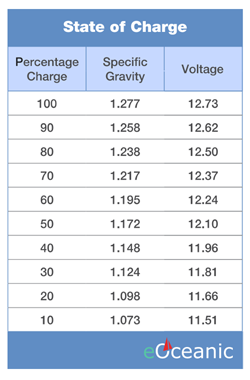
The voltage of a 12V battery bank should ideally be between 12 - 13 volts. A battery should rest a minimum of 30 minutes after charging to allow the surface charge to dissipate before a reading is taken. A fully charged 12V battery should register about 13.5 volts when there is no load on it, and this value should drop slightly when there is a load on it. Around 12.7 or 12.8 volts is a good average. The voltage of a 24V bank of batteries should be maintained at 24 – 26 volts offload and approximately 24V on load. The charging voltage should be more than 24V, usually 25 – 30 Volts, depending on the condition of the batteries which should be checked daily.
The problem with the float voltage is you have to completely disconnect any load from the battery and wait about 20 minutes for the battery to stabilize to take this reading. Add just the slightest load and a 12V battery will drop about 0.3 volt. Add more and it will fall further. This makes it difficult to know the precise capacity available depending on what loads are being imposed.
However with some experience of the various loads such as GPS, Navigation Lights etc, in conjunction with the aid of an accurate digital panel meter, you will in time find it is easy to get a good view of battery capacity in operation.
Hydrometers
 Hydrometer
HydrometerPhoto: Public Domain
The specific gravity will vary between 1,270 when fully charged and 1,150 when discharged. It is advisable not to allow the accumulate to discharge below 1,200 to prevent the formation of hard lead sulphate on the plates. Ideally, the specific gravity should be maintained at approximately 1,250 by regularly charging them or by keeping them on float-charge. In extremely cold or hot climates, batteries may be regionally specific and not have standard electrolyte (acid) strengths. The electrolyte may be stronger for cold, or weaker for very hot climates. In such cases, the specific gravity and the voltages may vary from what is indicated here.

Battery Monitors
The essential function of a battery monitor is to calculate ampere-hours (aHr) consumed and the state of charge of a battery. Ampere-hours consumed are calculated by detecting the current flowing in or out of the battery.
Battery monitors need to be programmed for different types of battery, some of which will have different levels of charging efficiency to others. For example, the charging efficiency of a flooded lead-acid battery is around 80% whereas that of a lithium battery is closer to 99%.
Battery monitors are available from companies such as Victron Energy and Nasa Marine. It is debatable whether a battery monitor provides a significantly better view of battery condition than does a simple voltage meter.

 The zero maintenance battery outcome
The zero maintenance battery outcomePhoto: Public Domain
- • The tops of the batteries and terminal connections to be dry, clean, free of dirt and corrosion.
- • That no objects are laying on top of the batteries
- • The battery casing is free from cracks.
- • That all vent caps are secured properly on the battery.
- • All the vents in filler caps are clean and open.
- • If fluids are present at the top of a flooded/wet battery this may mean that the battery is being over-watered. If fluid is on the top of a gel or AGM battery this means that the battery is being overcharged and the performance and life will be reduced.
- • That there is no corrosion build up on terminals - if they were not kept clean and dry.
- • That the battery cables and connections are clean and secure - battery problems are often caused by dirty or loose connections.
Before attending to any remedial work it is important to note all tools making battery connections are insulated with rubber handles and jewellery should be removed. Likewise keep sparks, flames and metal objects away from batteries and do not smoke in their immediate vicinity. It is always worth reminding oneself that you are working with corrosive acid, explosive gases and hundreds of amps of electrical current, that it is important to be attentive to the task. Typical maintenance steps involve:
- • Checking the fluid level should just cover the plates e.g. 5 mm. Battery plates exposed to air will immediately sulphate, however, do not be tempted to over-fill.
- • If you have no hydrometer to test the specific gravity of the battery, shine a torch into the filler holes and note the colour of the plates. A charged batteries will have a light brown positive plate and a slate grey negative plate. A discharged battery will have a whitish positive plate and a grey negative.
- • The plates should not be buckled.
- • Use only mineral-free water; distilled is best as all impurities have been removed and there is nothing left that could contaminate your cells.
- • Clean the top of the battery, terminals and connections with a cloth or brush and a solution of baking soda and water - a couple of tablespoons to ½ litre (pint) of water. But do not allow the cleaning solution to get inside the battery. Rinse with water and dry with a clean cloth.
- • Clean cable connections.
- • Replace any damaged cables with a replacement of the correct sizing.
- • Apply a thin coat of petroleum jelly (Vaseline) to the terminals to prevent corrosion.
- • Tighten any loose connections to the torque specified by the manufacturer making certain that there is good contact with the terminals. Do not over-tightening terminal connections as it can result in terminal breakage and loose connections which can result in meltdown or fire.
- • Coat the exposed cable end with petroleum jelly. The gases from the battery condensing on metal parts cause most corrosion.
- • Keep the area around batteries clean and dry.
Don't disconnect battery cables while the engine is running (your battery acts as a filter). While the battery is being charged, expect the plates to give off gasses causing bubbling, as it becomes fully charged, the amount of gas and bubbling increases.


All lead-acid battery based technology require a long charge time arising from a two-stage process: 'bulk' charge and 'float' charge. The first 80% can be 'bulk charged' within 2 or 3 hours, particularly so with AGM batteries that can handle a high bulk charging current. But then the charging current drops off dramatically as the 'float' phase commences and it typically requires another 9 to 10 hours.

This is not an issue if one is connected to the mains and charging overnight, but it is a huge issue if you have to leave the boat engine, or generator, running for hours on end as it can be rather noisy and expensive. It is all too easy to turn the engine off and end up with batteries that never actually get fully charged. Likewise, those relying on solar panels for the float charge can easily overlook the fact that solar power ceases when the sun sets and most likely before that final 20% has been topped off.
There is a further problem with using standard automobile alternators in a boating environment. The modern charging system hasn't changed much in over 40 years. It consists of the alternator, regulator, which is usually mounted inside the alternator, and the interconnecting wiring. The regulator keeps the charging voltage that the alternator produces between 13.5 and 14.5 volts whilst recharging the battery with a tapered charging process. The taper causes the battery to charge rapidly at first and then slow down as it reaches full charge. This eliminates the risk of overcharging and battery damage.
Unfortunately, this default setting is designed to replace automobile cranking amps which is a very specific use case and very different to the boating profile. This results in the regulator dropping the charge and choking off the 'bulk' charge potential unnecessarily early when charging a boat battery bank. This causes the engine to have to be run longer, adding more engine time, consuming unnecessary fuel and increasing the length the engine noise has to be endured in the cabin. Worse the auto regulator does not correctly comprehend deeply discharged batteries very well. This gives it a tendency to overcharge batteries that are very low which can damage batteries. So much so that it is estimated a deeply discharged engine-starting battery has, on average, only an estimated 10 deep cycles available when recharged by a standard alternator regulator.
Special smart three-stage chargers, or “intelligent” chargers, are available to optimise the charge of batteries. These typically use a 3-step charging technique:
- • Bulk Charge: During bulk charging, the charger is at the maximum voltage and current amp rating of the charger. For a typical 12 volt AGM battery, the charging voltage going into a battery will reach 14.6 - 14.8 volts, while flooded batteries can be even higher. For the gel battery, the voltage should be no more than 14.2 - 14.3 volts.
- • Absorption: When the battery voltage reaches 14.4 volts the 'absorption' charge' step commences. This is where the voltage is held at a constant 14.4 volts and the current (amps) declines until the battery is 98% charged.
- • Float: After this there comes the 'float' step. This is a regulated voltage of not more than 13.4 volts and usually less than 1 amp of current. This in time will bring the battery to 100% charged or close to it. The float charge will not boil or heat batteries, but it will maintain the batteries at 100% readiness and prevent cycling during long-term inactivity.
Use of a "smart" chip-controlled charger will optimise both battery performance and battery life and will pay for itself over the long haul. Refer to the instructions that came with your charging equipment. Most chargers are automatic and preprogrammed. Some chargers allow the user to set the voltage and current values and the below charger voltage settings for flooded batteries provide some guidelines that will serve in the absence of better information. If a smart controller is outside of the budget a very simple low-cost manual system can by creating with the help of an auto or marine electrician - see 'optimising the alternator's charging profile without an expensive 'smart' charger'.


The following charging guidelines are recommended:
- • Ensure sufficient capacity for the initial charge and it is optimised for the battery in use.
- • Replace the energy consumed immediately, or at the latest, within 24 hours of discharge. If you don't, the battery sulphates affecting performance and longevity.
- • Do not shorten the float charge as it can significantly reduce the life of the battery. Undercharging of a battery to only 90% of capacity will allow sulfation of the battery using the 10% of battery chemistry not reactivated by the incompleted charging cycle.
- • Do not charge at too high a rate. Don't let a battery get hot to the touch and boil violently when charging.
- • Do not run down batteries too low (voltage and specific gravity). Batteries can't stand deep discharge - see below.
- • Do not allow batteries to stand discharged too long.
- • Avoid charging at temperatures above 49°C (120°F)
- • Charge batteries daily.
Batteries should be kept at full charge when stored (or dry). A deeply discharged battery can freeze solid in sub-zero weather and should never be charged in a frozen state.

Marine batteries can last from between 1 - 6 years but it varies considerably depending upon how they are used, how they are cared for and the storage strategy adopted. The longest life of a standard lead acid battery, when working, is about the 48-month mark and it is achieved by keeping the depth of discharge of less than 20%. Marine deep cycle batteries may be discharged deeper in accordance to their individual specification but keeping them within the first of 20% discharge is always optimal.
Any cycle that has a discharge of over 80%, i.e. deeper than 20% or first ⅕, of the rated capacity is classed as a deep discharge. Each of these deep discharge cycles reduces the battery's lifespan and the deeper the discharge cycle the deeper the ageing of the battery and the fewer the number of cycles the battery can supply.

The above graph, courtesy of the Balqon Corp who are the Winston agents for North America, presents this key battery lifecycle to the depth of discharge, or DOD, equation very well. As can be seen, if a battery bank is drained down to no more than 20% every day of the year it is possible for it to provide a service life of around 3,000 cycles or approximately 8¼ years. But when the same battery is taken down to a depth of discharge of 50% every day their service life falls to a ⅓ of that lifespan or somewhere around 1000 cycles or 2¾ years. The Lithium battery at the same depth of discharge provides 5,000 hours, and at a daily depth of discharge of 70% provides 3,000 cycles showing the value of its performance not to mention weight to power.
However, there are vast differences in the cost, keeping inside the top 20% of the bank capacity is difficult when disconnected from the grid in the seagoing environment. So there is a trade-off between deeper discharge and long-term battery durability. For instance, the price of Lithium is exponentially more than lead-acid batteries. Likewise, a daily 50% depth of discharge will reduce the life of the batteries by approximately ⅔, but this profile delivers 150% extra capacity/utility, without additional weight, albeit at the cost of replacing the batteries every couple of years. This could be a more than acceptable service life for heavy users, especially if this is factored into the choice and price of the batteries selected.
A depth of discharge point that should not be overlooked here is how a parasitic drain can cause untold damage to a battery bank. Most boats have something such as an automatic bilge pump, radio, GPS, etc or as in my case, admittedly, a music sound system that operates without the engine running. If they are connected when the battery isolator switch is turned to 'off' they can take a non-charging battery bank down to zero in an unattended boat. Alternatively, a parasitic load may be caused by a short in the electrical system. If you are always having dead battery problems, most likely the parasitic drain is excessive. The constant low or dead battery caused by a parasitic energy drain will dramatically shorten battery life and should be attended to immediately. It is also worth noting that deeply discharging lithium batteries become unstable.
Vessels sailing to the tropics should also note that heat is an enemy to all these battery types. Nominal battery performance is usually specified for working temperatures somewhere between 20°C and 30°C. The performance and lifespan of a battery can be seriously affected by temperatures outside of this range, as the higher the battery temperature, the faster chemical reactions will occur and the colder, the slower. While higher temperatures can provide improved discharge performance, the increased rate of chemical reactions will result in a corresponding loss of battery life. As a rule of thumb, for every 10°C increase in temperature the reaction rate doubles. Thus, a month of operation at 35°C is equivalent in battery life is the equivalent of two months at 25°C.
Likewise lowering the temperature causes chemical reactions to proceed more slowly, so if a battery is used at a low temperature then less current is produced than at a higher temperature. In an engine start situation, the colder temperatures lowering of the power is compounded by an increasing thickness of the boat's engine oil, making the engine harder to turn over.
Equalization (Wet Cell)
Occasional charging at higher voltages, such as an equalizing charge, can benefit flooded lead-acid batteries by removing plate sulfation and refreshing the plates. This process is an “overvoltage overcharge” of 10% higher than the normal full charge voltage performed on flooded lead acid batteries after they have been fully charged. To maintain your battery in good condition you should equalize one or two times every year. Many 'smart' regulators have a special setting for equalization.
A safety precaution would be to equalize the battery for several hours, whilst aboard. Desulfation usually causes a bit of gassing, and so you should top up the fluid levels in wet cell batteries after running an equalization charge on them.

See Also
eOceanic's battery options for boating and electrical power generation on a sailing yacht provide a good outline to storage and power strategies. Below is a useful set of forun discussions:
- • Power & Electronics forum on Cruiser Log.
- • Electrical: Batteries Generators & Solar forum on Cruisers Forum.
- • Discussion thread regarding the replacement of electrolyte in lead acid batteries with sulphate salt solution on Cruiser Log.
- • Cruising Helmsman article on battery care.
- • See details on the Hydrometer on wikipedia.
Permission is granted to copy, distribute and/or modify this document under the terms of the GNU Free Documentation License, Version 1.2 or any later version published by the Free Software Foundation with author restrictions as may be seen on CruisersWiki.
With thanks to:
Delatbabel at the World Cruising WikiAdd your review or comment:
Please log in to leave a review of this tip.
eOceanic makes no guarantee of the validity of this information, you must read our legal page. However, we ask you to help us increase accuracy. If you spot an inaccuracy or an omission on this page please contact us and we will be delighted to rectify it. Don't forget to help us by sharing your own experience.

